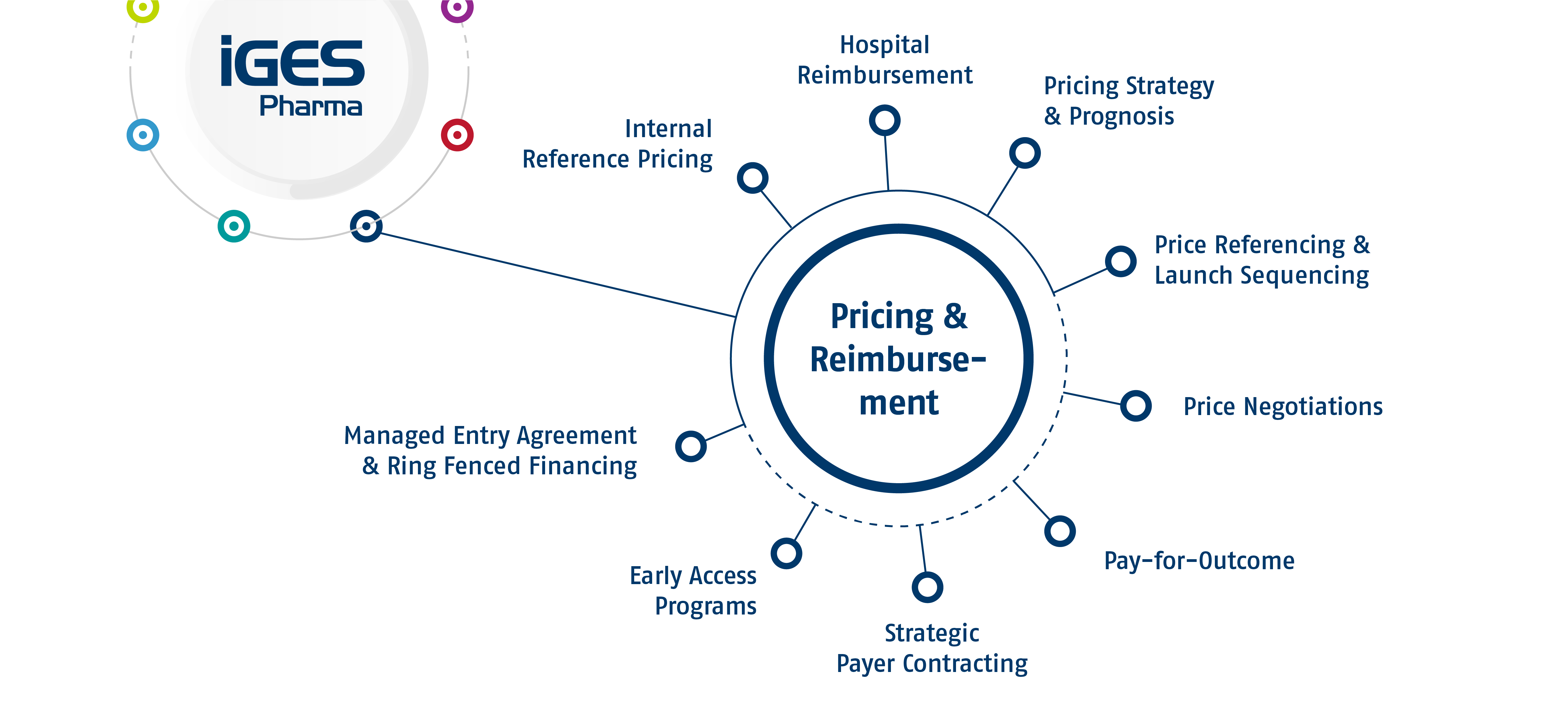
Pricing & Reimbursement
Finding the right price and ensuring patient access is key for the commercialization of your pharmaceutical product. IGES can analyze patient pathways both quantitatively or qualitatively to understand their position within their respective care setting. This way access hurdles can be identified and addressed. We can also support you early on with predictive data modelling to derive pricing estimates for your product or support your local price negotiations by doing the necessary research, deciding on a strategy, train you or even participate in the negotiations.
Products
Analysis of most suitable pricing strategy by considering product characteristics, current standard of care and future competition. Data driven modelling and insights through its network of current and ex-payers allow for estimation of realistic price assumptions tailored to product properties. Early predictions will cover different scenarios to account for uncertainites in assumptions (e.g. exact indication).
Price referencing & launch sequencing is key for successfully launching products across Europe. We understand the interdependencies of European price referencing and combine it with our experience of local HTA and P&R work to optimize each product’s launch sequence.
Preparation and strategic support of price negotiations in respective countries. Development of pricing scenarios, models and innovative payment contracts with payors. If desired, participation in the negotiation meetings and on-site support. Existing collaboration with specialized and well-known law firm. Coordination with strategic implications for launch sequence.
Analysis and overview of pay for outcome models. Product specific analysis of feasible models (measurement of outcomes, availability of data, contractual options). Considering alternative risk-sharing-models between payer and manufacturer as fall-back option for special circumstances. Analysis of risks and benefits as well as implementation of such models and assessment of suitable payment criteria.
Evaluation of market access options in case of challenges with the established HTA systems, for example in case of orphan drugs or conditional marketing authorizations. Examples include coverage under evidence generation, commercial agreements, or ring fenced financing instruments like the innovative medicines fund.
Selection of suitable health insurances in order to enforce individual contract agreements for the reimbursement of innovative pharmaceuticals. Engagement with selected payers/insurances. This also includes the preparation of the contract negotiations and, if desired, the participation.
Strategic analysis of early access programs and assessment of eligibility of product for early access program. Consideration of its implications for HTA/P&R.
Understanding current treatment of targeted patients in hospitals to assess impact on care for innovative pharmaceuticals. Analysing relevant diagnosis and procedures will hint at typical patient profiles (length of stay, associated treatments and comorbidities) as well as cost and reimbursement differences for cases of interest. Identification of (specialized) treatment centers to assess centralization of care and enablement of reimbursement for treating hospitals by navigating federal application processes.
Assessment of applicability of a product to internal reference pricing and development of strategies to achieve pricing in line with a products value proposition to healthcare systems. Identification of adequate analogue products with similar characteristics regarding targeted patient population, care setting, evidence package and HTA hurdles for validation or estimation of drug prices. Simulation of internal reference pricing and support for grouping and price-setting procedures.
 Contact
Contact